Rege Nephro will soon begin a clinical trial for autosomal dominant polycystic kidney disease (ADPKD) discovered by our scientific founder, Professor Kenji Osafune.

As announced on December 1, 2023, (https://www.regenephro.co.jp/en/news/2023-12-01/) a research group of our Director and Chief Scientific Advisor, Professor Kenji Osafune, succeeded in creating a disease model that reproduces the pathology of autosomal dominant polycystic kidney disease (ADPKD) using organoids(1) created from iPS cells, and discovered a potential therapeutic drug for ADPKD.
Rege Nephro Co., Ltd. (Head Office: Sakyo-ku, Kyoto, Representative Director: Akifumi Morinaka) announces that it will soon begin a clinical trial for this therapeutic candidate, a retinoic acid receptor (RAR) agonist.
ADPKD is an incurable hereditary disease in which numerous cysts gradually develop and enlarge in both kidneys, and kidney dysfunction progresses, leading to end-stage renal failure requiring artificial dialysis or kidney transplantation. In addition to the kidneys, cysts can also occur in the liver and pancreas, and it is known that cysts are frequently associated with systemic blood vessel abnormalities, high blood pressure, cerebral aneurysms, and heart valve abnormalities. Although the drug tolvaptan has been approved, there is no cure for ADPKD.
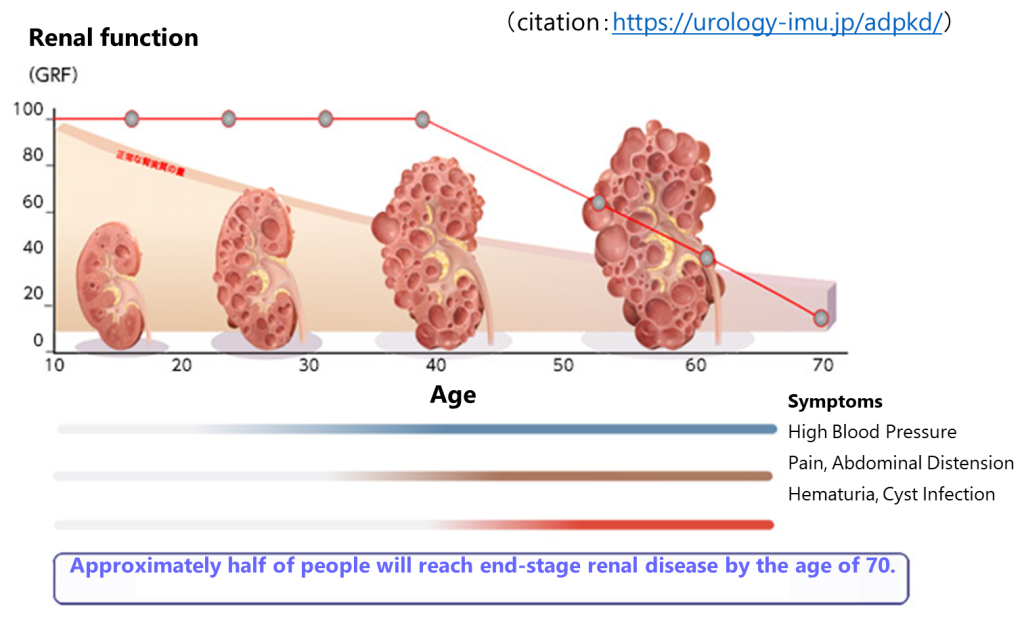

Until now, a disease model which has characteristics of the kidney nephron, a tissue that produces urine, had been created using human iPS cells. In this research, we succeeded in creating a disease model which has characteristics of a structure called a collecting duct that connects to the nephron and made great progress in elucidating the mechanism of ADPKD development and contributing to drug discovery. Cysts generally occur in the collecting ducts of patients with ADPKD, and the new disease model more accurately reproduces the pathology of ADPKD.
The RAR agonist was identified as a therapeutic candidate and is a drug that has been approved and used clinically for the treatment of relapsed and refractory acute promyelocytic leukemia (APL). Because the clinical trial uses an already approved drug, it will begin from the phase IIa(2), targeting patients with ADPKD who have a relatively high risk of disease progression and cannot be or does not want to treated with tolvaptan. We have already submitted the application for the clinical trial in early October. We plan to complete a clinical trial contract with a clinical trial facility in December and to begin accepting patient entries in early 2024.
Note (1) Organoid
A small organ created three-dimensionally in vitro.
Note (2) Phase IIa
A clinical trial that evaluates the efficacy and safety of a therapeutic candidate for a limited number of patients for which it is expected to be effective, and to collect information on the appropriateness of usage and dosage.